100 Stories1961 Why Fish Meat Is Not Salty - Research on Ion-Exchange Membranes
The development of ion-exchange membranes was triggered by an inspiration from Kagayaki Miyazaki, the president of Asahi Kasei. It happened when he and Yoshio Tsunoda, who later became president of Asahi-Dow, went to the United States and read a small newspaper article while they were in town.
Asahi Kasei's ion-exchange membranes now supply salt electrolysis processes to more than 160 plants worldwide and boast the top market share in China, the largest market. A simple moment and time led to the birth of the company's ion-exchange membranes.
When Tsunoda read that article and said, “It would be interesting to study fish skin,” Miyazaki felt a flutter of what could be called a manager's intuition.
He immediately gave the order to start research, but at first it was a continuous process of trial and error. As a result of his repeated research, he discovered, as an example, that by chemically treating synthetic resin membranes, it is possible to create membranes that allow either only cations or only anions to pass through.
He worked toward the industrialization of extracting salt from seawater by taking advantage of this property of these membranes. However, while the experiments at the Kawasaki plant were successful, they failed at Onahama, the site where the plant was to be built. The situation was so utterly confusing that some of the research team members even began to argue unscientifically that the seawater in Kawasaki and Onahama might be different.
Research and development expenses snowballed and exceeded 1 billion yen over 10 years. One billion yen at the time was a very large amount of money in today's money, and there was much criticism from within the company.
However, Miyazaki continued to back up the research team in anticipation of its future potential, and finally established salt production technology using ion-exchange membranes 11 years after the start of research. The salt manufacturing plant began operations in 1961, and today, Asahi Kasei's affiliates produce slightly less than 50% of all salt produced in Japan.
The next step was the production of sodium hydroxide. At first, the mainstream production method used two membranes to electrolyze brine to produce sodium hydroxide, but Miyazaki had doubts about the economics of this method.
He therefore ordered Maomi Seko, Asahi Kasei's leading expert on ion-exchange membranes and later president of Asahi Kasei, to reexamine the process. The result was not only the answer that Miyazaki had hoped for, but also the beginning of an unprecedented manufacturing process challenge.
They began work on the world's first production of sodium hydroxide using a single ion-exchange membrane. With Miyazaki's backing, the company's expectations mounted, and instructions to promote research and build a large-scale plant were passed down. The technical team led by Seko went through a series of hardships, but they succeeded in developing the product to meet expectations.
Today, Asahi Kasei exports this technology to companies around the globe, and the Asahi Kasei method accounts for about 60% of the world's production of sodium hydroxide using ion-exchange membranes.
Since the start of sales of the salt electrolysis process in 1975, the business was in danger twice due to downsizing through the NAC* program, in turn being downgraded from a full division to a business group. The ion-exchange membrane business overcame the hardships and turned into a profitable business in the late 2000s. This technology is not limited to the production of sodium hydroxide. Ion-exchange membranes have many applications in the desalination of seawater, recycling of wastewater, production of adiponitrile (a raw material for nylon 66), and in fuel cell membranes. This technology has a truly promising future.
NAC*: The “New Asahi Creation” Project which aimed to thoroughly review existing loss-generating projects, development projects, and R&D
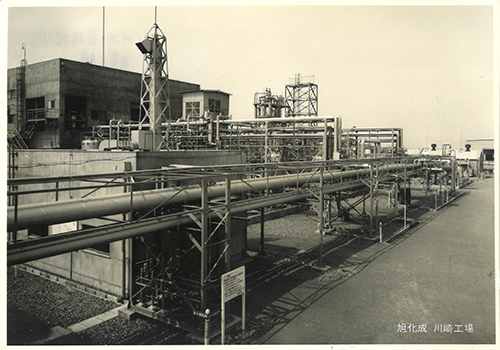 The ion-exchange membrane plant
The ion-exchange membrane plant
(Kawasaki,1960s)
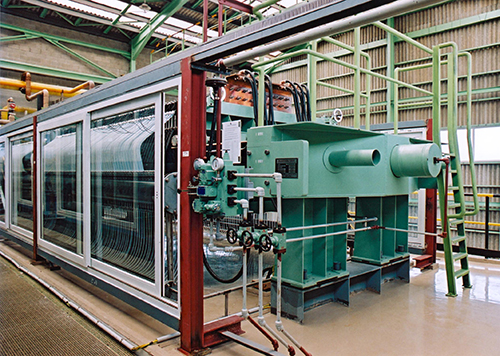 The electrolyzer with ion-exchange membrane
The electrolyzer with ion-exchange membrane

