Born in 1969 in Shizuoka Prefecture, Japan, Suzuki graduated from the Department of Fine Materials Engineering, Faculty of Textile Science and Technology, Shinshu University, and joined Asahi Kasei in 1991. He worked in the development of catalysts and process at the Mizushima petrochemical complex in Okayama Prefecture, and received a Doctorate of Engineering in System Science from Okayama University of Science in 2013. Current position since 2019. Suzuki’s research achievements have earned notable recognitions including the 2014 Chemical Society of Japan Award for Technical Development, the 2015 Chairman’s Award of the Japan Institute of Invention and Innovation, and the Development Category Prize for Science and Technology in the 2019 Commendation for Science and Technology by the Minister of Education, Culture, Sports, Science and Technology.
Interview with Dr. Ken Suzuki
Single-minded devotion to catalysts for industrial chemical innovation
A catalyst is a substance that promotes a chemical reaction without being transformed itself.
Catalysts lie at the core of many chemical processes. Dr. Ken Suzuki talks about how catalysts are fascinating, his turning point as a researcher, and the environment needed to create innovations.

Dr. Ken Suzuki
Principal Expert of Asahi Kasei Corporation
Senior General Manager, Chemistry & Chemical Process Laboratory, Corporate Research & Development
A catalyst is the essential core of a chemical process
A catalyst raises the speed of a chemical reaction or selectively promotes the intended reaction between different substances without itself being changed. A catalyst is a mysterious substance that acts as a match-maker, exchanging electrons between itself and the reactants, resulting in a new chemical compound.
In developing catalysts, it’s important to stick to the basics. I try to discern the nature of each catalyst from the earliest stage, in order to find one that is truly superior. Poring over the data, I anticipate the next outcome. There’s a knack to sensing whether a catalyst has potential or not. While that means being imaginative, I never lose sight of the final objective which is to use the catalyst in a large-scale chemical plant.

Gold nanoparticle catalyst for efficient, low-cost production of material for plastic
Although gold had previously been considered to be chemically inert, in 1987 a Japanese researcher reported that gold nanoparticles act as catalysts. Since then, gold nanoparticles have been the subject of attention around the world for the development of catalysts. Especially in Japan, applied research became very active with high expectations for practical application.
My team created gold–nickel oxide (Au–NiOx) nanoparticle catalysts with a core-shell structure having superior performance. In 2008 we succeeded in using them as the world’s first Au nanoparticle catalysts in a commercial chemical production process in a plant producing methyl methacrylate (MMA). The Au–NiOx nanoparticles have a core-shell structure, with Au nanoparticles at the core and the surface covered by highly oxidized NiOx. They are supported on a carrier with high dispersion. Asahi Kasei’s “direct oxidative esterification” (DIRECT METHA) production process for MMA using the catalysts has many advantages over the conventional process, including reduced consumption of energy and resources, no pollution, higher yield, less energy consumption, longer catalyst service life, greater safety, environmental compatibility, and superior economic performance.
The essence of this unconventional technology is to combine metal with metallic oxide at the nanometer-scale atomic level. Bonding dissimilar metals was considered to be difficult. But one day I happened to come across an unexpected phenomenon, and fortunately I discovered that gold and nickel oxide form a nanoparticle composite. Focusing on the phase structure, I succeeded in synthesizing the core-shell structure of bimetallic nanoparticle catalysts. My search for the best catalyst had been a series of failures, and I almost gave up. Step by step, however, I made progress, and when my hypothesis finally proved to be true, all of my hardship paid off.
 Au–NiOx catalysts. When gold is made into nanoparticles, its color turns purple. The color of purple stained glass comes from gold nanoparticles.
Au–NiOx catalysts. When gold is made into nanoparticles, its color turns purple. The color of purple stained glass comes from gold nanoparticles.
Environmentally friendly synthetic chemistry using CO2 as a resource
Leading the world with “green chemistry”
Being one of the top catalyst researchers, I was appointed in the first generation of Principal Experts two years ago. Currently, as Senior General Manager of the Chemistry & Chemical Process Laboratory, I am working with my team on R&D in the field of the environment and energy for a sustainable society. One of our current projects is to apply unique zeolites with a precisely controlled pore structure in the development of a system for efficient separation and recovery of CO2 from exhaust gases emitted from power plants and factories. As for CO2 conversion technology, we are commercializing technology to produce materials for polycarbonate (PC) and polyurethane, and also developing new technology to synthesize functional chemicals.
In the near future, when CO2 can be recovered at low cost and renewable energy is widespread, conversion technology by electrosynthesis using advanced electrocatalysts will become an important way to produce fuels and commodity chemicals. Asahi Kasei is the world’s first company to commercialize technology to produce PC using CO2 as a material. I am proud that Asahi Kasei is a pioneer in green chemistry to proactively use CO2 as a material, not just reducing CO2 emissions.
Researchers are creators; nonconformists and problem-solvers working together
You’ve got to be able to come up with new ideas free from preconceptions. In chemistry, I think it’s possible to get better at this with experience, unlike in physics. I tell my researchers to strike a balance between order and chaos. It can be fascinating when you don’t know what will happen, so I tell my people to keep going even if there is a lot of uncertainty. There’s nothing to learn from experiments that are too predictable. The things that everyone can agree on will most likely turn out to be unremarkable. Big discoveries come from peculiar ideas that most people wouldn’t think of. I’ve had that experience myself.
At Asahi Kasei, some eccentric characters are given the opportunity to spend time on their research if they seem capable. On the other hand we have people with excellent problem-solving skills, and we assign them tasks that make the most of their abilities. For the organization as a whole, I think it’s vital to have a balance between both kinds of people.

Young researchers plunging headlong with their own perspective and the perspective of chemistry
Our research is focused on building the next generation with innovative technology. This depends on the ability of each researcher. They all have different backgrounds, and different ways of working toward their goals. There are endless possibilities. I want each one to value their own experience, their own expertise, and their own intuitions that they build up over time. When people believe in themselves, and approach research seeking the true nature of things from their own perspective and the perspective of chemistry, creative ideas emerge. It might begin with a vague notion like daydreaming. But the pursuit of such ideas through the process of research can lead to new outcomes. Everyone needs to find the area of research that they are passionate about, something they want to pour all their energy into. When people are required to work on a research subject they don’t find interesting, I tell them to think of it as practice until they find their real field of interest. The experience of delving deeply into research will prove valuable in any field. So I strongly urge young researchers to plunge headlong into whatever tasks they have and learn to value the experience.
About Au–NiOx nanoparticle catalysts with core-shell structure for producing MMA
Au–NiOx nanoparticle catalysts with a core-shell structure for producing MMA was successfully used as the world’s first Au nanoparticle catalysts in a commercial chemical production process.
The Au–NiOx nanoparticle catalysts have a core-shell structure supported on a silica-based carrier, with the Au nanoparticles at the core and the surface covered by highly oxidized NiO. This structure of nanoparticles having a particular chemical state provides superior catalytic performance.
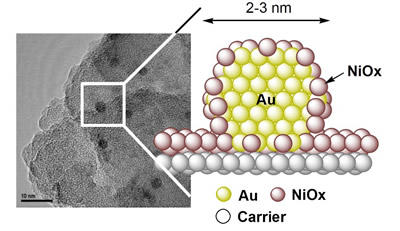 Typical transmission electron microscopy images of Au–NiOx nanoparticle catalysts and proposed structure of nanoparticles
Typical transmission electron microscopy images of Au–NiOx nanoparticle catalysts and proposed structure of nanoparticles
In 2008 these Au–NiOx nanoparticle catalysts with core-shell structure were successfully applied at the Kawasaki Plant, using the DIRECT METHA process for MMA production.
After ten years of commercial operation, these catalysts continue to provide superior catalytic performance with high selectivity, superior activity, and long service life, resulting in reduced consumption of energy and resources, and superior economic performance.
The direct oxidative esterification (DIRECT METHA) process is an original process of Asahi Kasei for the production of MMA. The process uses isobutylene as starting material, and MMA is synthesized by oxidation and esterification of methacrolein in a single step. This process was first used at the Kawasaki Plant in 1998.
 MMA production plant using Au–NiOx nanoparticle catalysts
MMA production plant using Au–NiOx nanoparticle catalysts
