Quality Assurance
Policy
Products and services provided by the Asahi Kasei Group internally and externally include materials, products, installations, various services, and after-sales support. We believe that providing safe and reliable products and services that satisfy our customers is our ultimate mission.
In 2016, we established the Asahi Kasei Group Quality Policy and Group Quality Assurance Bylaws. Based on these, we promote quality assurance to provide products and services that satisfy our customers and society.
As we enter an era of coexistence with the coronavirus and the post-coronavirus era, we have changed the Asahi Kasei Group Quality Policy on August 1, 2020, to reflect our strong awareness of the need to regard discontinuous and irreversible structural changes as opportunities for reform and to act on our own initiative.
Asahi Kasei Group Quality Policy
The Asahi Kasei Group flexibly anticipates the constantly changing needs of customers and society to create and provide products and services with quality that ensures safety and security.
Management Framework
In April 2019, we appointed a dedicated Executive Officer for Quality Assurance to further reinforce the management framework.
The Corporate Quality Ensurance department of Asahi Kasei Corporation oversees and coordinates Group-wide quality assurance activities.
Corporate Quality Ensurance consists of three groups: the Quality Ensurance Group, which supports the enhancement of each internal organization’s quality assurance activities; the Product Safety Group, which functions to ensure our product safety as a comprehensive chemical manufacturer; and the Quality Ensurance Planning Group, which proposes new plans and provides smooth connections between internal and external organizations. Corporate Quality Ensurance performs a head-office function as a hub for the Group's quality assurance framework and strives every day to reinforce quality assurance activities throughout the Asahi Kasei Group to deliver safe and reliable products and services to our customers and society.
Corporate Quality Ensurance prepares a Monthly Quality Assurance Report, based on which the Executive for ESH & QA and the Executive for Quality Assurance hold monthly quality assurance meetings to discuss information related to quality assurance.
Each core operating company and strategic business unit within the Group performs quality assurance in accordance with the products and services provided in each business area in conformity with uniform Group guidelines and bylaws.
The Group Quality Assurance Bylaws stipulate quality assurance activities for ESH & QA Administrators, such as the Presidents of the core operating companies and strategic business units, to lead. The bylaws also define the designation and roles of Quality Assurance Managers who play a central role in activities to enhance quality assurance. The Quality Assurance Managers' Conference is held four times a year to transmit and share information among the entire Asahi Kasei Group regarding quality assurance activities. In addition, from fiscal 2019, we have started to provide an opportunity for the Executive Officer for Quality Assurance and the Senior General Manager of Corporate Quality Assurance to meet directly with ESH & QA Administrators, Quality Assurance Managers, and others to discuss the enhancement of quality assurance and for frank exchange of opinions and sharing of ideas through face-to-face meetings, thereby creating an environment that enables us to build a reliable quality assurance system.
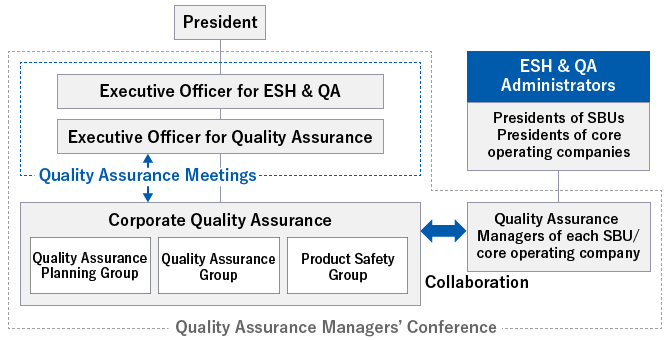 Diagram of quality assurance framework
Diagram of quality assurance framework- Quality Assurance Meeting (held each month):
- Based on the Monthly Quality Assurance Report prepared by Corporate Quality Ensurance, the Executive Officer for ESH & QA, the Executive Officer for Quality Assurance, and the Senior General Manager of Corporate Quality Ensurance hold the Quality Assurance Meeting to discuss information related to quality assurance.
- Quality Assurance Managers' Conference (held four times a year):
- Quality Assurance Managers, who play a central role in strengthening quality assurance, meet to transmit and share information among the entire Asahi Kasei Group.
Product safety initiatives
The Group has also formulated the Group Guidelines for Product Safety Measures in order to make the approach to product safety in the Group Quality Assurance Bylaws even more specific.
Product safety measures are implemented at each stage of product development and product supply, to ensure product safety and to prevent product problems and complaints from emerging. In addition, we have also established appropriate measures to be taken if a serious product problem or serious accident is likely to occur or if it does occur.
![[Product development phase] [Proposal] Preparation of measures to secure product safety, setting schedule → [Pre market-evaluation study] Evaluation of safety and risks, Elimination and reduction of risks, Identification of risks for users and risk reduction measures, Preparation of documentation, Identification of conditions for production and distribution → [Pre market-evaluation safety review] → [Pre-marketing study] Evaluation of safety and risks, Elimination and reduction of risks, Understanding methods of use and disposal, Identification of risks for users and risk reduction measures, Preparation of documentation, Confirmation and revision of conditions for production and distribution → [Pre-marketing safety review] → [Product supply phase] [Post-marketing study] Understanding methods of use and disposal, Revision of documentation, Application of conditions for production and distribution](/sustainability/social/quality/images/index-img-03.png) Flow of product safety measures
Flow of product safety measures
Safety assurance procedures
The procedures for realizing safe products and services are specified by the Guidelines for Ensuring Safety of Equipment and the Guidelines for Ensuring Safety of Chemicals.
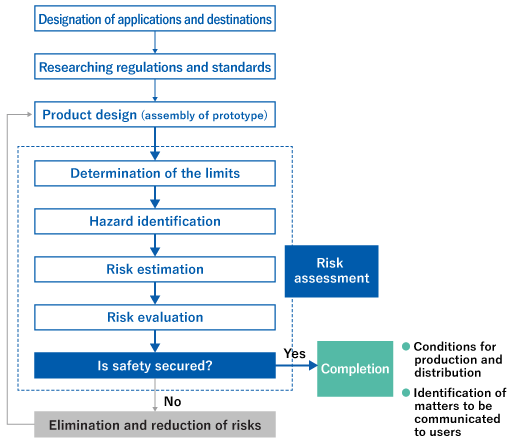 Product safety procedure for equipment
Product safety procedure for equipment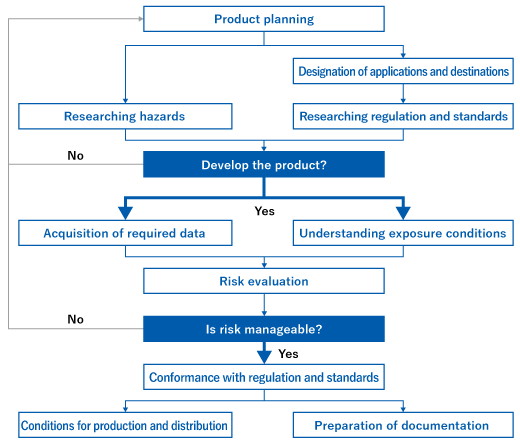 Product safety procedure for chemicals
Product safety procedure for chemicals
Chemical substance management
The Group identifies the properties of chemical substances and appropriately manages each process from product development, raw material procurement, and production (including intermediates) through to use and disposal in order to ensure the safety of products and production processes. We implement the chemical substance management shown in the diagram below at each stage from the perspectives of the global environment, operational safety, workplace safety, hygiene, and health, and quality assurance (product safety).
Corporate Quality Ensurance (Product Safety Group) serves as the secretariat for the implementation of chemical substance management in each business unit let by the Group’s Quality Assurance Managers.
-
![Society / Asahi Kasei Group / Green procurement, Global environment: Reduction of environmental burden, Operational safety: Prevention of fires and explosions, Physical distribution safety: Provision of Transport Emergency Cards / Supplier: [Supply]→[Material]→[Procurement]→[Production][R&D]→[Sale]→ Customer: [Product]→[Use]→[Disposal] / Receipt of SDSs for materials, Product safety: Distribution of SDSs for products, Workplace safety and hygiene: Prevention of workplace injuries](/sustainability/social/quality/images/index-img-06.png) Note: SDS stands for Safety Data Sheet.Chemical substance management flow
Note: SDS stands for Safety Data Sheet.Chemical substance management flow
R&D
The management of chemical substances begins in the R&D stage when the applications for chemical substances are determined, and is guided throughout every stage by a commitment to developing products and process characterized by safe, environmentally sound production, handling, and use. For products that are expected to be exported to other countries in the future in addition to being sold domestically, we conduct research on each country’s laws and regulations and consider the requisite measures.
Materials purchase
When purchasing materials, information related to the safety of chemical substances is received from the supplier. This information serves as a guide to safe storage and handling.
Production
At the production stage, we manage chemical substances, including intermediates, in an appropriate manner to suppress emissions into the environment. We also strive to prevent fires, explosions, and leaks at facilities where chemical substances are handled to ensure the safety of local communities and preserve the global environment. The health of employees is protected by performing sound risk assessment for chemical substances and preventing workplace exposure to hazardous substances.
Sale, use, and disposal
Guidance for proper use and disposal of chemical substances and chemical products is provided in Safety Data Sheets (SDSs), technical bulletins, and product brochures.
Transport Emergency Cards are issued to guide the proper environmental and safety response in the event of an accident during physical distribution. Moreover, when products are exported outside of Japan, we take appropriate measures to comply with laws and regulations, such as complying with the EU REACH regulation.
Quality assurance and human resources development
Development of core human resources for quality assurance
We have held the Quality Assurance Forum since fiscal 2017 to continue heightening awareness of quality assurance among younger and mid-level employees across the Group. In fiscal 2022, 50 employees selected from throughout the Group gathered once per month for a period of six months for a group discussion on a certain subject each time, combined with lectures on each subject by outside experts (mainly university professors at the forefront of the Japanese Society for Quality Control). At the final session, participants in each business sector discuss issues in their own organizations based on what they learned, and prepare proposals to present to management.
In fiscal 2023, we are continuing this as a key project for in-house quality assurance training.
Fostering a quality assurance mindset among department and group managers
In fiscal 2022, we invited an expert lecturer from outside the company to hold a seminar on mental well-being for department managers to further deepen their understanding of how to create an organization with a culture of openness. In addition, a Quality Assurance Seminar for Managers was held to enhance on-site manufacturing.
Quality assurance training for department and group managers is slated to continue in fiscal 2023 and beyond.
Fostering a quality assurance mindset among all employees
Coinciding with Quality Month in November 2022, the President, the presidents of core operating companies, the president of each SBU, and the Senior General Manager of Corporate Quality Ensurance issued messages for all Group employees about the importance of quality assurance. Training via e-learning was conducted as well, further raising awareness on the subject for employees.
This is slated to continue in 2023 and beyond as well.
Chemical substance management training
We provide regular training to research, production, and sales staff in each area of the Group. Such training includes sharing the most up-to-date information on the latest domestic chemical substance-related laws and regulations (Act on the Evaluation of Chemical Substances and Regulation of Their Manufacture, etc., Industrial Safety and Health Act, Poisonous and Deleterious Substances Control Act, etc.) in Japan and overseas for the management of chemical substances and consideration of responses, and presentations of the latest themes in chemical substance management.
Appropriate labeling and information provision
Providing appropriate information to our customers
The Group, which provides customers with products and services that are end products for domestic and household-use products, provides information that includes product performance, precautions, and suggested usage to ensure safe use of our products and services.
We endeavor to provide descriptions of products and services to customers, including product labeling and advertisements, that are easy to understand and not misleading. In addition, we confirm the content of descriptions and advertisements of products and services at each stage from product development and introduction to sale, and continuously check that there is no infringement of related laws, regulations, or voluntary industry standards, and confirm that customers are able to properly use products and services safely and reliably.
Compliance with the revised Food Sanitation Act
In June 2020, the revised Food Sanitation Act came into effect, and a new positive list (below “PL”) system was introduced.
The Group participates in a number of committees of the JCII (Japan Chemical Innovation and Inspection Institute) Food Contact Material Safety Center, and it is continuing its activities to ensure that nothing is overlooked in the PL system and to provide customers with appropriate information related to the PL system.
Responding to Globally Harmonized System (GHS)
Globally Harmonized System of Classification and Labelling of Chemicals (GHS) is a system for classifying and labelling chemicals in accordance with globally unified rules in order to help with accident prevention and health and environmental protection. The Group is advancing a program to classify the hazards of all of our chemical products in accordance with GHS categories, and revise our SDSs and label our products with safety information accordingly.
Compliance with chemical substance regulations around the world and sharing of information
As laws and regulations concerning chemical substances continue to be adopted around the world, such as the REACH Regulation* in the EU, we have been confirming, responding, and managing within the company to ensure compliance with them. In addition, some of these regulations require the sharing of information. Besides providing the necessary information to our customers, we actively work to provide information on chemical substances contained in products throughout the supply chain. One of our activities is participating in the Joint Article Management Promotion-consortium (JAMP) as an upstream company since its establishment in 2008. We continue to promote the use of chemSHERPA, a communication tool, as part of JAMP’s activities.
- * Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) is a regulation in Europe on chemical substances. It obliges registration of the usage and safety of chemical substances imported to or produced in Europe. Substances judged to pose high risks are subject to authorization and restriction.
Mechanisms to utilize customer feedback
We believe satisfying customers and providing products and services that are a delight to use translates into contribution to society. In order to achieve this, we believe that it is most important to identify true needs by listening carefully to customer feedback to establish two-way communication. The Group has built frameworks for such communication with customers in each of our businesses and strives to listen to frank and honest feedback.
![[Materials, intermediates, devices] [Asahi Kasei Group] Sales and R&D departments, Fibers & textiles, chemicals & polymers, construction materials, electronic materials & devices, medical devices [Customers] Processors, manufacturers, trading companies (→ Response / ← Inquiries, suggestions, requests) / [Final products, homes] [Asahi Kasei Group] Customer support center of each product (Customer Support Center, Hebelian Center, Medicine Consultation Line, etc.) Saran Wrap™ cling film, Ziploc™ bags & containers, Hebel Haus™ homes, pharmaceuticals [Customers] Consumers (→ Response / ← Inquiries, suggestions, requests)](/sustainability/social/quality/images/index-img-07.png) Communication with customers
Communication with customers
