Interview with Dr. Akira Yoshino
Development history and future outlook of lithium-ion battery
The lithium-ion battery (LIB) is essential to many of the products we use every day. The person who invented the LIB is Dr. Akira Yoshino of Asahi Kasei.
What motivated your research on the lithium-ion battery?
There was a lot of R&D on portable electronics in the 1980s, and so small and lightweight batteries, with high energy density and rechargeability were also needed. But nobody really knew what kind of rechargeable battery was going to be needed. The big buzzword at first was “portable,” soon joined by “cordless” and “wireless.” I just sort of sniffed out the direction that trends were moving. You could say I had a good sense of smell.
How do you come up with new ideas for R&D?
I try to consider what people need, what the world really needs, based on my own experience in daily life. Then I think about how technology can be a means to accomplish it. I’ve found that it’s more likely for a good technology idea to pop into my head when I’m relaxing, with a clear mind, rather than when I’m concentrating hard trying to think of something.
Could you explain the essence of your invention? How has it influenced society in general?
Previously, rechargeable batteries used water as the solvent for the electrolyte. However, water is electrolyzed into hydrogen and oxygen when the voltage is over 1.5 volts. For that reason, getting more than 1.5 volts was practically impossible. So I used organic solvent instead of water, and using carbon as negative electrode I was able to get over 4 volts. With lithium cobalt oxide as positive electrode material, I created the world’s first LIB.
The commercialization of the LIB as a small and lightweight rechargeable battery contributed greatly to the development of portable electronic devices such as mobile phones, laptop computers, digital cameras, video cameras, and portable music players. The LIB is increasingly used in electric vehicles, helping to expand a new market.
 The first prototype LIB cell, made in 1983
The first prototype LIB cell, made in 1983
How do you think fields of research will change in the future?
The IT Society we live in today resulted from the IT Revolution which began in 1995, the year Windows 95 was launched. Everything has changed dramatically since then, and the world of today would look like a sci-fi film if viewed from the perspective of 1995.
I suppose such revolutions will happen again, in another 10, 20, or 50 years. While the IT Revolution occurred in the field of information, I believe the next revolution will be in the field of energy. Preparations for the upcoming revolution are already advancing. One thing that never changes is that scientists who clearly grasp society’s emerging needs and boldly take on new research challenges will be the leaders who open the path to the future.
Lastly, do you have a message for future scientists? Could you tell us about your aspirations for the future?
Since we live in a society flooded with so much information, it may be hard for young scientists to appreciate that there are many fields where unknown things are waiting to be discovered. There are many opportunities for groundbreaking R&D. With a clear objective and persistent effort, the possibilities are endless. As for me, I intend to remain on the front line of research, taking on challenges in new fields.
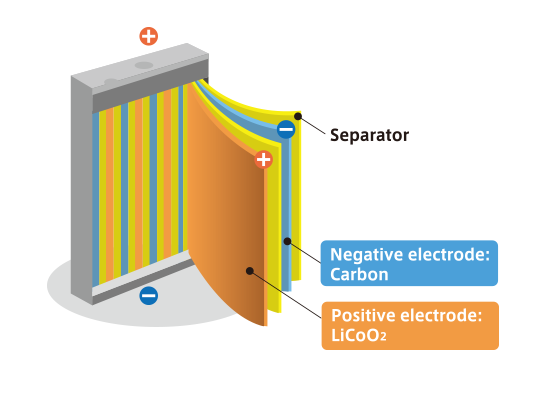
What is the LIB?
The LIB is a rechargeable battery having a metal oxide compound containing lithium as the cathode (positive electrode) and carbon as the anode (negative electrode). As the battery is charged and discharged, lithium ions move back and forth between the cathode and anode. It is used in many different applications today, including smartphones, laptop computers, vehicles, industrial machinery, and airplanes.
Characteristics of the LIB
- 1.High electromotive force and high capacity enabled through the use of nonaqueous electrolyte and particular combination of materials for positive and negative electrode.
- 2.Significantly heightened battery safety by avoiding use of metallic lithium which has high chemical reactivity.
- 3.Under charging, lithium ions are released from the positive electrode and migrate into the carbonaceous material of the negative electrode. The reverse reaction occurs during discharging, and electric energy is stored or released by repeating these reactions reversibly. Utilization of a cell reaction method without chemical transformation, which provides excellent cycle durability over a long service life.
Types of battery
| Aqueous electrolyte battery (cell voltage 1.5 V max) | Nonaqueous electrolyte battery (high voltage/high capacity) | |
|---|---|---|
| Primary battery (disposable) |
Manganese dry cell, Alkaline dry cell |
Metallic lithium battery |
| Secondary battery (rechargeable) |
Lead-acid battery, Nickel-cadmium battery, Nickel-metal hydride battery |
Lithium-ion battery |
Operating principle
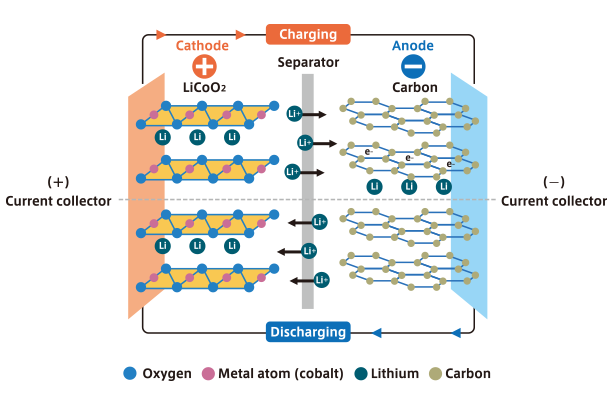
Structure of the LIB